EDTA Acid, EDTA Powder
CAS No.: 60-00-4
Molecular Formula: C10H16N2O8
Molecular Weight:292.24
Structural Formula:
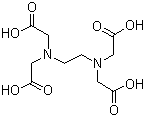
Properties:
EDTA acid (CAS 60 00 4) is a white crystal powder. EDTA acid can be soluble in sodium hydroxide, sodium carbonate, and ammonia solution. However, EDTA powder is not soluble in cold water, acid, and common organic solvents and is discomposed at 240℃.
Q: What is the solubility of EDTA at pH=0 or lower in an acidic aqueous solution?
I want to measure the total amount of EDTA in an aqueous solution, but various divalent and trivalent ions have been complexed with EDTA. It is said that EDTA is almost insoluble under acidic conditions, so it is thought that if EDTA does not dissolve at pH = 0 or lower, then EDTA can be weighed out by lowering the pH value to achieve the purpose of measuring the total amount.
A: Under alkaline conditions, EDTA can form monosodium, disodium, trisodium, and tetrasodium salts and dissolve in water, but its solubility is not large. Under acidic conditions, both monosodium and disodium are acidic, but they are soluble in water.
Only ethylenediaminetetraacetic acid has low water solubility. At this time, the PH needs to be tested. Specifically, the PH of the test shall prevail, and the adjustment shall be carried out to achieve the best precipitation value. The external acid will form a salt with the amino group to increase the solubility, so the system can not have cations such as sodium ions nor anions such as hydrochloric acid (in other words, EDTA is soluble in hydrochloric acid). Therefore, the best and maximum precipitation value is the pH value of EDTA itself, which can be tested by putting EDTA in water, preferably with an acidometer.
Specifications:
| Item | Index |
| Appearance | White Powder |
| Purity | ≥99.0% |
| Chloride (Cl) | ≤0.05% |
| Sulfate (SO4) | ≤0.05% |
| Iron (Fe) | ≤0.001% |
| Heavy metal (Pb) | ≤0.001% |
| Chelate value (mgCaCO3/g) | ≥339 mgCaCO3/g |
| pH | 2.8~3.0 |
Usage:
EDTA acid is mainly applied to complex metal ions and separating metal. It can be used as a water-treating agent and a scale-eliminating agent. EDTA can prevent the harmful effects on production caused by metal ions in water. This chelant is also used in cleaning boilers in large-scale power plants.
EDTA is widely used as a bleaching and fixing solution for color photographic material processing, dyeing auxiliary, fiber treatment auxiliary, cosmetic additive, blood anticoagulant, detergent, stabilizer, and synthetic rubber polymerization initiator.
EDTA acid is a representative chemical of a chelating agent. It can form stable water-soluble complexes with alkali metals, rare earth elements, and transition metals. In addition to sodium salts, there are ammonium salts and iron, magnesium, calcium, copper, manganese, zinc, cobalt, aluminum, and other various salts. Each of these salts has different uses. For details, you can check the related products on the website.
EDTA can also detoxify the body from harmful radioactive metals by rapidly excreting them.
Ethylene Diamine Tetraacetic Acid is an important indicator for titrating metals like nickel and copper. It should be noted that when used with ammonia, it is an indicator.
Packing and Storage:
Packed in paper bags lined with plastic; net-weighted 25kg per bag; stored in cool, dark, ventilated, and dry places.
Keywords:
Ethylene Diamine Tetraacetic Acid. EDTA-4H.
-
EDTA Series
- EDTA Acid
- EDTA-Na2
- EDTA-Na4
- EDTA-CaNa2
- READ MORE...
-
Organophosphoric
- ATMP
- BHMTPMPA
- DTPMPA
- EDTMPA
- HEDP
- READ MORE...
-
Other Chelating Agent
- DTPA
- NTA.Na3
- DTPA.Na5
- READ MORE...







